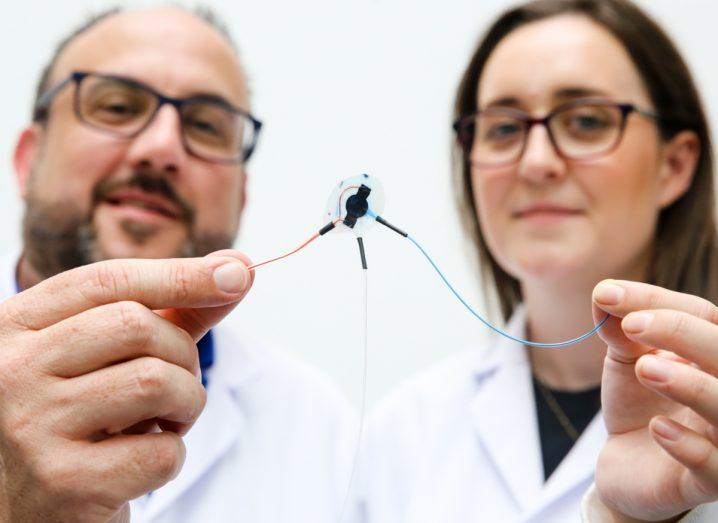
From left: Prof Garry Duffy and Dr Rachel Beatty. Image: Martina Regan
An implant created by a team from the University of Galway and MIT can deliver personalised doses of a drug for extended periods of time.
Implantable medical devices have offered promise in unlocking advanced therapeutic interventions in healthcare, such as insulin release to treat diabetes. But the human body’s adverse reaction to foreign objects has been a major stumbling block.
Now, a team of scientists from the University of Galway and Massachusetts Institute of Technology (MIT) have developed a novel implantable device that can remain in a patient’s body for extended periods, releasing drugs as and when required.
“Imagine a therapeutic implant that can also sense its environment and respond as needed using AI,” said Dr Rachel Beatty of the University of Galway. “This approach could generate revolutionary changes in implantable drug delivery for a range of chronic diseases.”
Beatty is the co-lead author of a paper based on the study that led to the breakthrough device, which was published in the journal Science Robotics yesterday (30 August).
The transatlantic team originally developed first-generation flexible devices, known as soft robotic implants, to improve drug delivery and reduce fibrosis.
However, these devices did not account for how individual patients react and respond differently, or for the progressive nature of fibrosis, where scar tissue builds around the device, encapsulating it, impeding and blocking its purpose, and eventually forcing it to fail.
“I wanted to tailor drug delivery to individuals but needed to create a method of sensing the foreign-body response first,” explained Beatty.
This is when the team deployed an emerging technique called mechanotherapy that helps reduce scar tissue formation with soft robotic implants that make regular movements – such as inflating and deflating – in the body.
A machine learning algorithm was also developed and deployed to predict the required number and force of actuations to achieve consistent drug dosing, regardless of the level of fibrosis present.
Combined with computer simulations, this method helped the researchers explore the potential of the device to release drugs over time with a surrounding fibrotic capsule of different thicknesses.
“If we can sense how the individual’s immune system is responding to an implanted therapeutic device and modify the dosing regimen accordingly, it could have great potential in personalised, precision drug delivery,” said co-author Prof Ellen Roche of MIT.
“[This ensures] the right amount of drug is delivered at the right time. The work presented here is a step towards that goal.”
Funded by Science Foundation Ireland’s AMBER and Cúram research centres, the EU Horizon 2020 and MIT, the study has resulted in a device that senior author Prof Garry Duffy describes as having worked out “the best regime to release a consistent dose”.
“We showed a worst-case scenario of very thick and dense scar tissue around the device, and it overcame this by changing how it pumps to deliver medication,” explained Duffy.
“Our discovery could provide consistent and responsive dosing over long periods, without clinician involvement, enhancing efficacy and reducing the need for device replacement because of fibrosis.”
10 things you need to know direct to your inbox every weekday. Sign up for the Daily Brief, Silicon Republic’s digest of essential sci-tech news.